
Nano Biomechanics & Mechanobiology Laboratory
Principal Investigator: Dr. Scott Wood





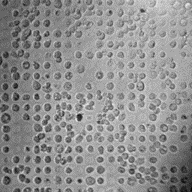
One major limitation hindering the scientific community's ability
to develop treatments to reverse osteoarthritis (OA) is that chondrocytes
de-differentiate after approximately 10 days in culture under standard
monolayer conditions. Micropatterned discs of printed proteins have shown some utility
for preventing de-differentiation but they do so by un-naturally restricting
cell adhesion. Although chondrocyte phenotype can generally be maintained for
much longer in 3D cultures, these methods severely limit analytical techniques
for assessing cellular behavior due to optical and diffusion-based limitations.
To combine the best of both worlds, we are developing the CellWell -
a novel micropatterned platform to prevent chondrocyte de-differentiation
in a fully adherent culture while maintaining compatibility with traditional
cell culture and analytical techniques. These abilities will make the
CellWell a potentially powerful tool to efficiently study the pathogenesis
of osteoarthritis at the cellular level.
Based on the results of our recent NSF-funded I-Corps project, we are also working
with corporate collaborators, and in association with the recently established
Center for Drug, Disease, and Delivery Governor's Research Center (3D Center),
to expand upon the concepts that inspired the design of the CellWell to develop a
modular 'joint on a chip' platform that can model the complex multi-tissue structure
of human joints. We expect this new platform to increase the translational potential of
in vitro (i.e., cell culture-based) preclinical drug development research to
treat joint diseases, including OA, helping bring life-changing discoveries from the
benchtop to the bedside more quickly, while maximizing safety and efficacy.
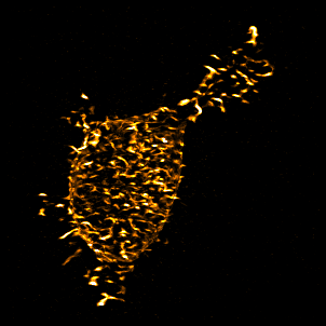
Articular chondrocytes are very mechanically sensitive cells, responding differently to
forces at different magnitudes as well as to forces of the same magnitude applied at
different frequencies in ways that either prevent or lead to osteoarthritis (OA).
Despite this and the known importance of their cytoskeletal integrity, which distributes
forces throughout the cells, surprisingly little is known about how chondrocytes respond
to forces exerted upon them by their environment or about what governs their differential
response to forces at different frequencies. We are working to determine how the motion and shape
of the actin cytoskeleton influences the frequencies and magnitudes of intracellular force
transmission through various integrins to regulate the homeostatic expression of anabolic
and catabolic factors.
As part of this project, we have developed an exciting method based on techniques commonly
used in product design engineering that allows us to quantify the motion of cells across a
wide range of physiological and pathological frequencies. We hope that this technique will
shed new light on the contributions of mechanobiological proteins across a wide variety of
cell types. One fun application of this technique is that we can plug the frequencies and
amplitudes we observe into drum synthesizer software to allow us to 'hear the motion' of cells.
Here is a brief example of what the motion of integrins of a chondrocyte bound to fibronectin would
sound like if we imagine it as though the cell was banging on a drumset: